Galectin Therapeutics focuses on studying the science of galectin proteins and applying this knowledge in the clinic. Galectins are important therapeutic targets because they are believed to be the mediators of fundamental biologic mechanisms in many pathological processes.
Galectins are a class of proteins made by many cells in the body, but normally most concentrated in immune cells. As a group, these proteins are able to bind to sugar molecules that are part of other proteins in and on the cells of our body. Galectin proteins act as a kind of glue, bringing together molecules that have sugars on them.
There are 15 galectin protein subtypes with the common characteristic of a carbohydrate recognition domain that binds to galactose-containing carbohydrates and glycoproteins. Galectin-1 and galectin-3 are the two most prominent galectins involved in pathological processes. A key factor that differentiates galectin protein subtypes is their oligomerization domains that allow the galectin protein to bind with another protein of the same family.
Galectins are widely studied, with nearly 3,500 scientific publications with “galectin” in the title. Galectin Therapeutics has edited two peer-reviewed books that bring together articles on the function of galectin proteins in disease. View the book, Galectins and Disease Implications for Targeted Therapeutics, here.
Secreted galectins have been shown to bind with high affinity to galactose-containing glycoproteins on the cell surface and in the extracellular matrix. While they all bind to glycoproteins with terminal galactose residues, each galectin binds to different sets of glycoproteins in the body thus providing specificity of action. Their ability to dimerize creates the opportunity for galectins to link glycoproteins and form a lattice structure on the cellular surface and to promote cell-cell and cell-matrix interactions. See image below.
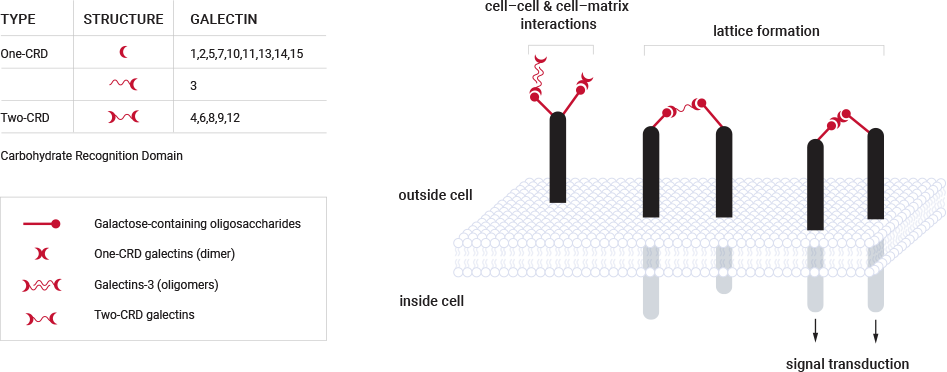
The associations between glycoproteins created by galectins can trigger intracellular signaling and potentiate or modulate certain biologic and pathologic effects. Galectin proteins also have intracellular functions, but these functions rely on protein-protein interactions and not interaction with glycoproteins. Galectin Therapeutics is focused on the extracellular functions, which are accessible to targeting with our compounds in development.
Learn more about the structure and function of galectin proteins in this Biochemistry review article.
Galectin proteins are known to be markedly increased in a number of diseases, including scarring of organs and cancers of many kinds. The increase in galectin protein promotes the disease and is detrimental to the patient.
While galectins are normally expressed in small amounts in many different cell types, they are most highly expressed in macrophages of the immune system. Secreted galectins are markedly increased and have important roles in a wide variety of pathological processes involved in immune regulation, inflammation, fibrogenesis and tumor cell biology. Galectins have been implicated in diseases as diverse as endometriosis and Alzheimer’s.
Galectin Therapeutics is developing compounds that are designed to bind to and inhibit the function of secreted galectins. With these characteristics, our drugs have the potential to modulate and treat diseases where increased galectins promote or potentiate the pathological situation. Learn about our compounds.
