In the liver, fibrosis is the end result of multiple inflammatory conditions and infections. Progressive liver fibrosis leads to scarring (cirrhosis), which results in reduction of liver function, multiple medical complications and ultimately death. Nearly 500,000 patients have cirrhosis in the United States with close to 50,000 losing their lives yearly. Only a fraction of patients’ lives were saved by liver transplantation at a cost of $350,000 per transplantation.
We believe that Galectin Therapeutics’ drug candidates have the potential to treat forms of liver fibrosis. All diseases that affect the liver lead to the development of scaring of the liver. Scientific evidence strongly suggests that galectin-3 is essential for the development of liver fibrosis in animals.
One condition in particular that frequently leads to cirrhosis is non-alcoholic steatohepatitis (NASH or fatty liver), a liver disease characterized by the accumulation of fat in the liver with associated inflammation and fibrosis which can lead to end-stage cirrhosis requiring liver transplantation.
The National Institute of Health estimates that 9 to 15 million Americans are affected by fatty liver disease and forecasts that the number of Americans affected by this disease is growing due to obesity and diabetes, with the potential to become the leading cause of liver cirrhosis and liver transplantation in the future.
Liver transplantation is currently the only therapeutic approach to fatty liver disease or other forms of liver fibrosis as there are no drug therapies on the market. Organ transplantation is a difficult, risky and costly procedure as organ availability is scarce and there is the risk of developing cirrhosis in the transplanted liver from the same disease that damaged the patient’s original liver. Therefore, there is a great need for other therapeutic options.
Galectin Therapeutics has used an animal model which is closely analogous to that of fatty liver disease in humans, in that the mice were given diabetes and then subsequently fed a high-fat diet, both conditions associated with the human disease. The mice develop a liver disease that is identical to that in humans with fat laden hepatocytes, ballooning degeneration of hepatocytes, inflammatory cell infiltrates and deposition of collagen (fibrosis).
View our data in fatty liver disease and our fatty liver disease development program.
The liver plays a central role in clearing chemicals from the body and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses and sometimes even when introduced within therapeutic ranges, may injure the liver. Drug-induced liver injury often leads to acute liver failures.
We believe that data indicate that treatment with GR-MD-02 is able to reverse and prevent the development of scar tissue in the liver, thus affecting toxin-induced liver fibrosis.
View our research in toxin-induced liver fibrosis.
Traber PG, Zomer E (2013) Therapy of Experimental NASH and Fibrosis with Galectin Inhibitors. PLOS ONE 8(12): e83481. doi:10.1371/journal.pone.0083481
Non-alcoholic steatohepatitis and resultant liver fibrosis is a major health problem without effective therapy. Some data suggest that galectin-3 null mice are resistant to the development of NASH with fibrosis. In the preclinical study published in PLOS ONE, NASH-induced mice were treated with GM-CT-01 and GR-MD-02 at two different points – early fibrosis and later more severe fibrosis. The studies evaluated twice-weekly, dose escalation of once weekly by intravenous administration, as well as evaluated different routes of administration including intravenous, subcutaneous and oral. Results revealed that treatment with GR-MD-02 significantly improved NASH activity and reduced fibrosis including prevention of accumulation of collagen and/or reduced accumulated collagen in the liver. Similar effects were seen with GM-CT-01 but with approximately four-fold lower potency than GR-MD-02. The data also show reduction in galectin-3 expression and other inflammatory biomarkers.
Data presented at the Annual Meeting of the American Association for the Study of Liver Diseases in Boston in November 2012. Abstract: “Galectin-3 targeting drugs inhibit multiple pathological pathways leading to improvement of non-alcoholic steatohepatitis (NASH)” Hepatology, Volume 56, 867A, 2012. View poster presentation.
These data showed that that following four weeks of treatment with GR-MD-02 there was a significant reduction in fat accumulation, hepatocyte degeneration and inflammation in the liver histology and improvement using a standard NASH grading system. Additionally, the percent of collagen in the livers (fibrotic tissue as demonstrated by percent Sirius red staining) was reduced by treatment with GR-MD-02 to levels equivalent to normal levels regardless of whether treatment was started early (before fibrosis developed) or late (after the development of fibrosis). These effects in the NASH mice were independent of serum glucose and lipid levels, which were elevated in all animals.
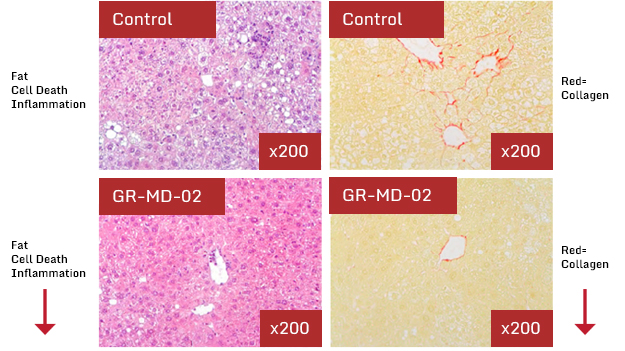
Traber PG, Chou H, Zomer E, Hong F, Klyosov A, et al. (2013) Regression of Fibrosis and Reversal of Cirrhosis in Rats by Galectin Inhibitors in Thioacetamide-Induced Liver Disease. PLOS ONE 8(10): e75361. doi:10.1371/journal.pone.0075361
Multiple experiments have been done in a toxin-induced model of liver fibrosis and cirrhosis in rats. In the preclinical study published in PLOS ONE, fibrosis was induced in rats by injecting thioacetamide (TAA) into the abdominal cavity. Rats were then treated with GR-MD-02 (galactoarabino-rhamnogalaturonan) or GM-CT-01 (galactomannan). In the initial part of the study, rats that completed eight weeks of thioacetamide injections were given four weeks of treatment with GR-MD-02; results showed an almost 50 percent reduction in collagen content, a marker of chronic fibrosis. Rats were then exposed to additional thioacetamide injections and developed extensive fibrosis (cirrhosis); treatment with four once weekly doses of GR-MD-02 or GM-CT-01 while continuing treatment with the toxin TAA led to marked reduction in fibrosis and reversal of cirrhosis. Overall, the study demonstrated that GR-MD-02 or GM-CT-01 led to significantly reduced fibrosis, reversal of cirrhosis and a significant reduction in portal hypertension.
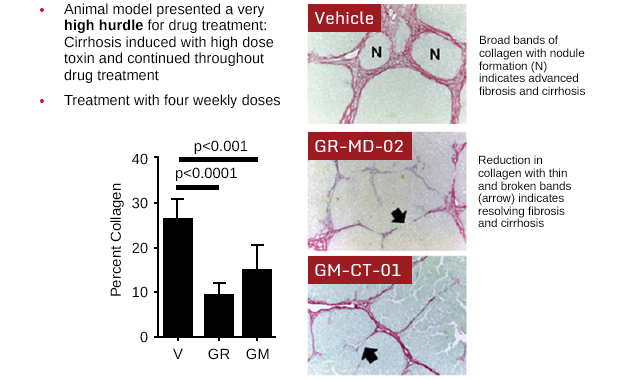
Data presented at the World Liver Congress of the European Association for the Study of Liver Diseases in Amsterdam on April 27, 2013. Abstract: “Regression of fibrosis and reversal of cirrhosis in thioacetamide-induced liver fibrosis following treatment with galectin inhibitors” Journal of Hepatology, Volume 58, page S462-3, 2013. View poster presentation.
Rats were treated with a toxin at a dose and duration to induce cirrhosis, the most advanced form of fibrosis. The vehicle-treated animals (controls) had severe fibrosis and cirrhosis with nodule formation (N). Following four weekly injections of either GR-MD-02 or GM-CT-01, there was marked reduction in collagen, thinning and breakage of bridging necrosis (arrows), resolution of nodules, and reversal of cirrhosis.
